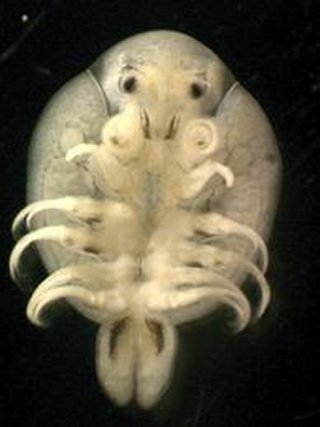
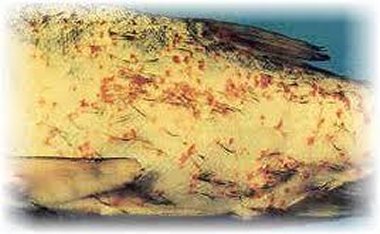
In North America, Lernaea spp. infect a number of cultured freshwater fishes. In summer, in waters with temperatures ranging from 25 to 28 ° C, this parasite finds excellent conditions for reproduction utilizing a number of fish cultured for food and ornamental purposes.
An interesting case of unusual fish mortality caused by L. cyprinacea was published by Goodwin (1999). During June and July of 1998, at least 3 Arkansas fish farms polyculturing bighead carp (Hypophthalmichthys nobilis) with channel catfish (Ictalurus punctatus) suffered major losses of channel catfish associated with massive infections by L. cyprinacea.
The catfish had few adult Lernaea attached to their skin, but there were from 8-50 copepodids on the surface of each catfish gill filament (Figs. 1, 2).
The copepodids were grazing on the gill tissue, and their feeding activity was associated with gill damage including epithelial hyperplasia, telangiectasis, and hemorrhage. Catfish skin was also covered by copepodids (Fig. 3).
Bighead carp in the same ponds were reported to have had numerous adult copepods on their skin but did not die during the epizootic. It is possible that the filter-feeding apparatus of the carp captured the copepodids thus preventing heavy infection of the gill filaments (Goodwin 1999). Lernaea copepodids have not been implicated previously in fish losses resulting from parasite damage to gills.
The loss of catfish in these cases is likely to have been due to their being polycultured with the bighead carp, a species that is a common host for adult lernaeids. There is no treatment for Lernaea infections that is legal for use with food fish. Most farmers have quit polyculturing these 2 fish species.
Lernaeids also cause problems for small baitfish and ornamental fish species. Arkansas produces millions of dollars worth of minnows each year. These fish are marketed when they are only 5 cm long. Lernaeid infections are common, and a single parasite in a critical location is enough to kill a minnow. In goldfish and koi, lernaeids cause some mild infections, but disfigure the fish making them unsuitable for sale. The primary method for control of this species in non-food fish is the pesticide Dimilin (diflubenzuron).
Lernaea cyprinacea was introduced into South America in the beginning of the 20th century via the importation of the common carp, Cyprinus carpio. Since then, the copepod has spread very quickly, is now a common parasite infecting all farmed species in Brazil, and is also very common in wild fish in all main drainage basins throughout the country. Gabrielli and Orsi (2000) studied 8 fish species on 53 fish farms of the State of Parana for the presence of lernaeids.
All fish species were infected, but 100% prevalence was found only in C. carpio, Leporinus macrocephalus, and Prochilodus lineatus. Wild fishes in rivers were infected at low intensities. In Brazil, lernaeids cause mortality in several species of farmed fish. Another issue is the presence of parasites in a growing number of“fish-and-pay”enterprises. At fish-and-pay facilities, anglers pay for the privilege of fishing in well-stocked ponds (usually earthen ponds).
The demand for this kind of leisure activity has led to the construction of thousands of fishand- pay sites in Brazil. The presence of fish parasites, especially lernaeids in fish-and-pay ponds, and the uncontrolled movement of fish throughout the country pose serious health threats to the fish farm industry (Pavanelli et al. 2000). Lernaea cyprinacea has also been recorded on cultured fishes on Caribbean islands (Fajer et al. 1985).
Another area relatively recently conquered by lernaeids is Australia (Hall 1983, Rowland and Ingram 1991, Langdon 1992). Heavy infections with Lernaea sp. resulted in mortality of golden perch (Macquaria ambigua), Murray cod (Maccullochella peeli), and silver perch (Bidyanus bidyanus) broodstock held in ponds (Callinan 1988). In Oct. 1978, there was a heavy infection of Lernaea sp. on Murray cod broodfish in 2 ponds at a research station following the use of common carp as a forage fish.
Replacement of carp by goldfish (Carassius auratus) as a forage fish seemed to stop the problem (Rowland and Ingram 1991). This suggests that carp is an important host to Lernaea sp. and may be responsible for the spread and increased prevalence of infections in native fish (including those cultured in ponds) (Rowland and Ingram 1991).
Carp were introduced to Australia more than a century ago and have since reached such a substantial biomass that they are considered a pest not unlike rabbits are on land.
Carp are blamed for spreading, not only lernaeids, but also the pathogenic tapeworm, Bothriocephalus sp., and other disease agents.
A recent survey in New South Wales showed that the incidence of externally visible abnormalities of fish was correlated with the density of carp In Africa, rapid increases in Lernaea burdens have been observed in association with growing environmental stress in some areas. High prevalences of“anchor worm”were recorded by Oldewage (1993) in tilapia of Lake Victoria.
The 2nd major branch of freshwater cyclo- poids parasitic on fishes is represented by the genus Lamproglena spp. These copepods typically are gill dwellers, and as such they have the potential to cause fish losses in aquaculture. Lamproglena comprises more than 40 nominal species (Piasecki 1993a).
They occur in Africa (Marx and Avenant-Oldewage 1996, Ibraheem and Izawa 2000), Asia (Kuang and Qian 1985, Kumari et al. 1989), and Europe (Caki´ c et al. 1998, Galli et al. 2001). To date, no fish kills caused by Lamproglena spp. have been reported.
The family Ergasilidae (Poecilostomatoida) comprises 24 valid genera (Amado et al. 1995) with 249 nominal species (The World of Copepods 2002). The overwhelming majority of species occurs in freshwater environments. The morphology of ergasilids largely resembles that of free-living cyclopoids, but some may be extensively transformed, e.g., Mugilicola.
The best known ergasilids are representatives of the genus Ergasilus, which contains 153 nominal species (The World of Copepods 2002) and more than 80 valid species (Kabata 1985). The best known species is Ergasilus sieboldi, which is 1.7 mm long and attaches to fish gills using its 2nd antennae.
The antennae, transformed into powerful hooks, hold the gill filaments tightly and can cause tissue damage and obstruct blood flow. Parasites feeding on epithelial cells stimulate hypertrophy and consequently a coalescence of secondary gill lamellae. This in turn drastically reduces the surface available for gas exchange. Lesions on gills are often attacked by secondary pathogens such as bacteria and fungi.
Feeding of E. sieboldi was described in detail by Einszporn (1965a b). This particular species attaches to the outside of the gill allowing some of its congeners to explore the space between the gill filaments. In cases of extremely heavy infections of whitefish, the parasite attaches not only to gill filaments but also to the fins (Kozikowska 1975). The life cycle comprises 6 nauplius stages, 5 copepodid stages, and adults. Males die after copulation, while females remained attached to the fish host (Abdelhalim et al. 1991).
In Central Europe, the 1st spring generation of E. sieboldi becomes sexually mature in mid- June. Their eggs hatch and the copepodids attack fish. They mature and produce a 2nd generation in September. Sometimes a 3rd generation follows by the end of the season. One female can produce 200 offsprings. Theoretically, the ensuing 2nd generation can comprise 40 000 descendants and in the 3rd, as many as 8 million (Schäperclaus 1992). Ergasilus sieboldi is not host-specific and can infect a majority of freshwater fishes; however the tench, Tinca tinca, appears to be the most susceptible.
This fact is attributed to the sluggishness of this fish, which may make it more vulnerable to copepod attack. Other less-infected fishes include pike (Esox lucius), bream (Abramis brama), whitefish (Coregonus lavaretus), vendace (Coregonus albula), carp (Cyprinus carpio), and roach (Rutilus rutilus).
Authors:
Wojciech Piasecki, Andrew E. Goodwin, Jorge C. Eiras, Barbara F. Nowak