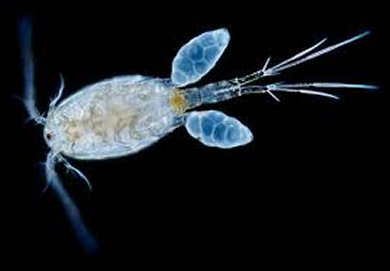
Some cyclopoids are micropredators of fish larvae, and especially vulnerable are the early stages of cyprinids (e.g., carp) due to the small size of the young fish. Fish larvae are attacked by adult copepods (e.g., Acanthocyclops robustus) and by more-advanced copepodid stages.
The results are serious lesions of the fins, blood vessels, yolk sac, head, nares, and particularly the gills (Fabian 1960, uromska 1967a b, Lillelund 1967; Kabata 1970, Fritzsche and Taege 1979, Hartig et al. 1982, Schäperclaus 1992, Mamcarz 1990). Piasecki (2000) documented the process of predation and its results on fish larvae, noting that mortality rates depend on the cyclopoid density and on the availability of alternative food (e.g., rotifers) for copepods.
If copepods have enough rotifers to feed on, they tend not to harm fish larvae. The most frequent fish attackers were mature males (33%), copepodids IV (29%), and copepodids V (22%). Females usually fed upon the already killed larvae (Piasecki 2000).
The State of Arkansas in the US produces a lot of cyprinids and hybrid striped bass. These species are stocked in ponds as small fry and are very vulnerable to attack by cyclopoids. Farmers use several strategies to avoid this problem.
The first is to time the filling of the pond and fertilization so that the fry can be stocked prior to the development of large copepod populations. Another strategy is to use Dylox (trichlorfon) to kill predatory copepods just prior to fish stocking. However, some copepod micropredators may be beneficial to fishes in that they consume copepod fish parasites.
Kasahara (1962) observed that some freeliving copepods such as Mesocyclops sp. prey on free-swimming larvae of Lernaea sp. Some freeliving copepods are also enemies of mosquito larvae and can reduce numbers of these insects in aquaculture waters (Marten et al. 1994).
Fish parasites:
The cyclopoid family Lernaeidae is represented by freshwater parasites that are highly adapted to a parasitic way of life. The majority of lernaeids have undergone extensive morphological adaptations hiding their close affinity with their freshwater cousins of the genus Cyclops. Fish parasites within this family belong to 14 valid genera (Ho 1998) with 180 nominal species (The World of Copepods 2002) and about 110 valid species (Ho 1998).
Representatives of the genus Lernaea have been studied more intensively than any other freshwater copepod group due to its economic importance. Lernaeids are probably the best known copepod parasites, and examples appear in most invertebrate zoology textbooks. There are 105 nominal Lernaea species (The World of Copepods 2002) of which only about 37 are valid (Kabata 1979).
Poddubnaja (1973) complicated understanding of the species concept of lernaeids by producing different phenotypes, resembling different described species of Lernaea from a single maternal specimen.
Lernaea species occur on all continents, with the majority in Africa. The only cosmopolitan species is Lernaea cyprinacea, which can infect a variety of freshwater fishes. Originally, L. cyprinacea was not present in South America and Australia, but it was accidentally introduced there with cyprinids.
Female Lernaea are highly metamorphosed vermiform ectoparasites (not mesoparasites) without segmentation that reach lengths of 12-16 mm (plus an additional 6 mm of egg sacs). The head, equipped with“antlers”that anchor the parasite in the subdermal tissues of a host fish, earned the parasite its vernacular name of“anchor worm”.
The rest of the body and egg sacs protrude into the water. This particular way of attachment is very pathogenic by its nature (Dzidziul 1973, Khalifah and Post 1976, Kabata 1985, Shariff and Roberts 1989). Initially the skin and muscles adjacent to the head become hyperemic, swollen, and susceptible to secondary infections. The parasite, s attachment evokes severe acute inflammation.
The host , s connective tissue reacts to the parasite, forming a thick fibrotic capsule around the anchor. Lernaea spp. can cause severe fin damage. Wounds caused by the parasite , s implantation occasionally develop into fistulae, penetrating the visceral cavity, including the heart and sometimes resulting in peritonitis and death (Kabata 1985). Considering the invasive method of attachment and severity of the associated damage, it is surprising that Lernaea-induced fish kills are not common (Shariff and Roberts 1989).
Feeding and the gut structure of Lernaea were described by Sabatini et al. (1988). The life cycle comprises 3 nauplius stages, 5 copepodid stages, and adults (Grabda 1963a). Copepodids settle on fish, mature, and copulate; and then males die and females undergo transformation while attached permanently to the host. Lernaea spp. are warm-water parasites, and according to Schäperclaus (1979) in some areas of the United States, no fewer than 10 generations can appear in the course of a year.
The history of fish mortalities caused by Lernaea spp. goes back to 1880, when in one of the lakes of the Masurian Lake District (presently in Poland), lernaeosis almost wiped out an entire population of the crucian carp (Benecke, cited by Kocy owski and Mi czy´ nski 1960). According to Kocy owski and Mi czy´ nski (1960) a mass mortality of crucian carp was also reported by Kozikowska in Karasiowe Lake, Poland and by Grabda in Wilczak Hatchery, Poland.
The copepod intensities reached 40 parasites per fish. An interesting account of site selection by adult L. cyprinacea was published by Dorovskikh (1996). In recent years, the numbers of Lernaea have drastically declined in Central Europe. In Poland, there has been no published record of Lernaea within the last 30 years although more than 280 papers dealing with fish parasites were published during that period (Piasecki and Woli´ nski, unpubl. data).
Authors:
Wojciech Piasecki , Andrew E. Goodwin , Jorge C. Eiras, Barbara F. Nowak